Primary care, urgent care, and hospitals demand fast, reliable PT/INR testing for oral anticoagulation therapy (OAT). That’s why Siemens is introducing the Xprecia Stride™ Coagulation Analyzer, a truly handheld device that tests PT/INR with lab accuracy.
The Xprecia Stride analyzer is ergonomically designed, with a large, intuitive touchscreen to enhance the user experience, simplify work flow, and improve overall testing accuracy. Together, these features make the testing process seamless and help minimize errors.
Get trusted results for critical analytes with ease, efficiency, and economy.
The RAPIDLab® 348EX Blood Gas System* is the cost-effective solution for smaller laboratories tasked with the challenge of performing fast-turnaround critical care tests. Fully-automated operation supports low-to-medium throughput on an easy-to-use analyzer that’s ready to generate accurate, on-demand results when clinicians need them, with a minimum of operator involvement.
Increase productivity and operator efficiency in the low-volume laboratory
The RAPIDLab 348EX analyzer combines proven Siemens blood gas technology with a range of next-generation system enhancements designed to streamline and simplify day-to-day operations and workflow.
Fast, accurate results support critical treatment decisions
- Actionable patient test results are available in approximately 60 seconds
- Small sample size (50 µL–95 µL) suitable for most patients
- Analyze whole blood and dialysate fluid
- Whole blood results: pH, pCO2, pO2 Na+, K+, Ca++/Cl–, and hematocrit
- Dialysate results: pH, pCO2, Na+, K+, Ca++, HCO3–, and ctCO2
- Test and report only those parameters ordered by the clinician
Ensure data integrity and increase workflow efficiency
- Bar-code reader, for patient/operator ID entry, can be mounted on either side and offers optional setting for continuous-on, single-handing scanning
- Sample probe aspirates from syringes, capillaries, and QC ampules automatically, without the need for adapters
- Automatic detection of short samples shifts analyzer into microsample mode, with no operator involvement required
- Time-tested Ready Sensor® electrodes have a proven track record of performance and long use-life
- Comprehensive QC materials help verify system performance
- Automatic calibration routines and wash sequences minimize demands on operators time and attention
Easy-to-use with fast access to sensors, reagents, and waste
- Intuitive, color, touch screen user interface features large, easy-to-read system icons that display analyzer status
- One-touch icon selection of sampling mode – syringe, capillary, dialysis fluid, QC
- Easy navigation to all routine functions from the Ready screen
- Measurement chamber houses all sensors and is designed for easy access and replacement
- All reagents are located on the front panel of the analyzer, enabling easy reagent replacement
- Clear plastic bottles and blue-colored solutions allow at-a-glance checks of fill volumes
- Minimal maintenance requirements further extend system uptime
Affordable, on-demand performance from a compact design
- Economically priced – low cost of acquisition
- Low operating costs – cost of reagents/consumables will not vary significantly, even if throughput triples from five to 15 samples/day
- Small footprint saves valuable bench space in smaller labs and point-of-care settings
Flexible data review, reporting and storage
- Results can be viewed on-screen, output to the onboard printer, or electronically transmitted to LIS/HIS or RAPIDComm® Data Management System
- Convenient onboard storage of up to 250 patient test records, and up to 90 results for each level of QC
- Print calibration and QC summaries for inspection purposes including off-line generated Levey-Jennings reports to document QC performance
- USB port simplifies data uploads/downloads
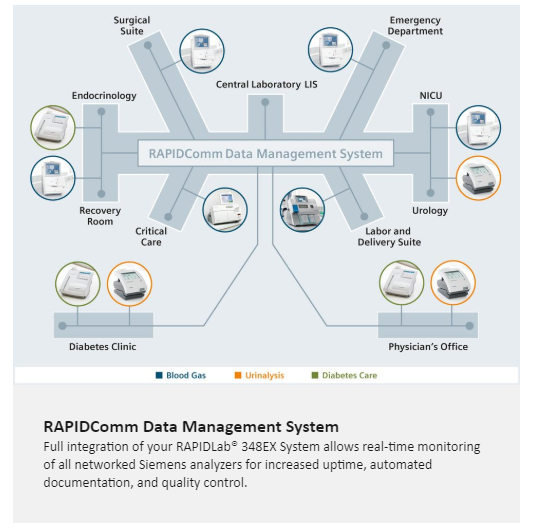
Increase efficiency with the RAPIDComm Data Management System
When analyzers in remote, decentralized settings are supported by the RAPIDComm Data Management System, point-of-care coordinators can oversee the entire testing process from a central location.
- Configure, monitor, and control multiple networked RAPIDLab 348EX analyzers
- Troubleshoot analyzers, standardize test protocols, authorize and certify operators, and enforce QC checks
- Generate customized reports for accreditation inspections
- Help ensure testing and regulatory compliance, and improve risk management
- Connect multiple Siemens point-of-care analyzers – including blood gas, urine chemistry, and diabetes care systems — through a single interface to the LIS/HIS
| Measurable Parameters | alyte | ||
Blood Gas | pH pCO2 pO2 | ||
Electrolytes | Na+ K+ Ca++ or Cl– | ||
Hematocrit | HCT | ||
| Calculated Parameters | O2SAT O2CT HCO3–act HCO3–std ctCO2 BEb BEecf, pO2(A-a) pO2 (a/A) pO2/FiO2 Ca++(7.4) Anion Gap |
Renal Dialysate | This mode permits measurement of Na+, K+, Ca++, pCO2 and pH in bicarbonate-based or acetate-based renal dialysis fluids. |
| System Overview | |
|---|---|
| Sample Size | Syringe: 95 µL Capillary: 95 µL Microsample mode: 50 µL |
| Sample Type | Whole blood Dialysate fluid |
| Analysis time | Approximately 60 seconds to result |
| Calibration | Automatic or on demand |
Input Parameters
Temperature: | 10.0°C–43.9°C | |
| Hemoglobin: | 2.0–25.0 g/dL | |
FIO2: | 15.0%–100.0% | |
| Patient/Operator IDs: | Up to 16 characters (alphanumeric) | |
| Sample location: | Radial, brachial, femoral, cord, arterial line (with RAPIDComm® Data Management System) | |
| Display Interface | Intuitive, icon-based color touchscreen |
|---|
| Onboard Computer | |
|---|---|
Storage Capacity/Memory | Up to 250 patient test records Up to 90 results for each level of QC |
| Data Export | Via integrated USB port/flash drive to PC, or direct to LIS/HIS or RAPIDComm® Data Management System |
| Connectivity Options | |
|---|---|
| Serial | RS232, LIS1, LIS2, LIS3 protocol |
| Bi-directional | Can be set up for monitoring/control and connectivity via the RAPIDComm Data Management System |
| Peripheral Interfaces | ||
|---|---|---|
| USB Port | Standard USB 2.0 | |
| External Barcode Reader | Standard USB 2.0 | |
| Environmental | |
|---|---|
| Temperature: | 15ºC–32ºC |
| Humidity: | max. 85% at 32ºC non-condensing |
| Barometric Pressure | 400-825 mmHg |
| Power Requirements | ||
|---|---|---|
| Rating: | 80 VA | |
| Voltage: | 100–240 V | |
| Frequency: | 50/60 Hz | |
| General | ||
|---|---|---|
| System Dimensions | Width: Depth: Height: Weight : | 385 mm (15.2 in.) 353 mm (13.9 in.) 382 mm (15.0 in.) 9.4 kg (20.7 lb) |
| Approvals | UL, CSA, IEC, EN-61010, CE Marked with full CB Scheme and all National Deviations. Complies with the IVD directive |
|---|